Project SPINET (Germany)

Neutrophil extracellular traps (NETS) orchestrate disease progression in patients with spondyloarthritis or diffuse idiopathic skeletal hyperostosis (DISH).
Research group:
Dr. Annika Heuer, University Medical Center Hamburg-Eppendorf
Prof. Michael Boettcher, University Medical Center Mannheim, University of Heidelberg
Dr. Jasmin Knopf, University Medical Center Mannheim, University of Heidelberg
Prof. Martin Herrmann, University Hospital Erlangen
Axial spondylarthritis (axSpA) and diffuse idiopathic skeletal hyperostosis (DISH) are prevalent conditions with serious clinical implications. The term axSPA describes inflammatory arthritis affecting the spine and/or the sacroiliac joints. It leads to variable degrees of pain, disability, and, in severe cases, complete loss of spinal flexibility. Moreover, it poses a risk of spinal fracture even after trivial traumata, in particular of the cervical spine due to increased rigidity combined with spinal osteoporosis. DISH is a common spinal enthesopathy with a largely unknown disease etiopathogenesis. It is associated with mechanical, dietary and low-grade inflammatory diseases resulting in symptoms like pain, limited spinal motion, and increased susceptibility to unstable spinal fractures even from minor traumata. The spinal immobility in both axSpA and DISH exacerbates inflammation-related bone loss, further elevating fracture risks, although the precise mechanisms are still elusive.
Neutrophils are the predominant leukocytes of acute inflammatory reactions and are the main innate immune cells involved in the early phases of wound healing. In response to infection and/or injury, these cells release their DNA associated with histones and cytotoxic proteins like neutrophil elastase (NE) or myeloperoxidase (MPO) and form web-like structures referred to as neutrophil extracellular traps (NETs). While NETs are crucial for bacterial clearance and resolution of inflammation, they can also exacerbate several inflammatory conditions. They are involved in the etiopatho¬genesis of many diseases as mediators of immunothrombosis. Furthermore, NETs and their degradation products damage adjacent tissues and prolong inflammatory processes. Once formed, NETs are usually cleared by DNases and subsequent phagocytosis and macropinocytosis. If this clearance fails or is insufficient, NETs persist and gradually change their appearance and composition in a process referred to as NET ripening. There is evidence that NETs together with fibrin and thrombin can form a composite network mutually stabilizing each other. Interestingly, both fibrin and DNA (as present in NETs) have been shown to induce bio-mineralization.
In this research project, we are investigating the role of NETs and NET-fibrin-co-aggregates in the formation of ectopic calcifications that chronify the inflammation in axSpA and DISH, thereby driving pathological bone remodeling. To this end, patients are recruited and biopsies are collected at the University hospitals Hamburg and Mannheim. The analyses and quantification of NETs and their degradation products in tissue and serum will be performed in the University clinic Erlangen and the German Center for Immunotherapy in Erlangen.
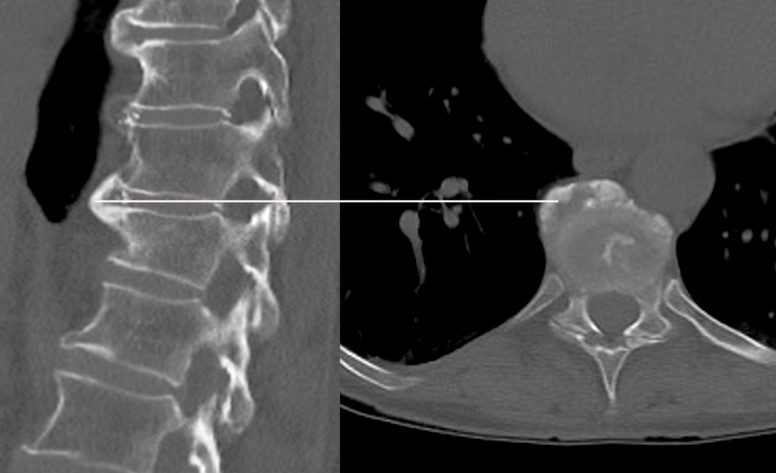 Figure 1 CT images of a patient with DISH in the thoracic spine.
Figure 1 CT images of a patient with DISH in the thoracic spine.(left) side view, (right) anterior-posterior (a.p.) view. A white line marks the coronal plane, which intersects the spine at the level of noticeable ventral hyperostosis. The latter is characterized by bone growth bridging two spinal vertebrae endplates.
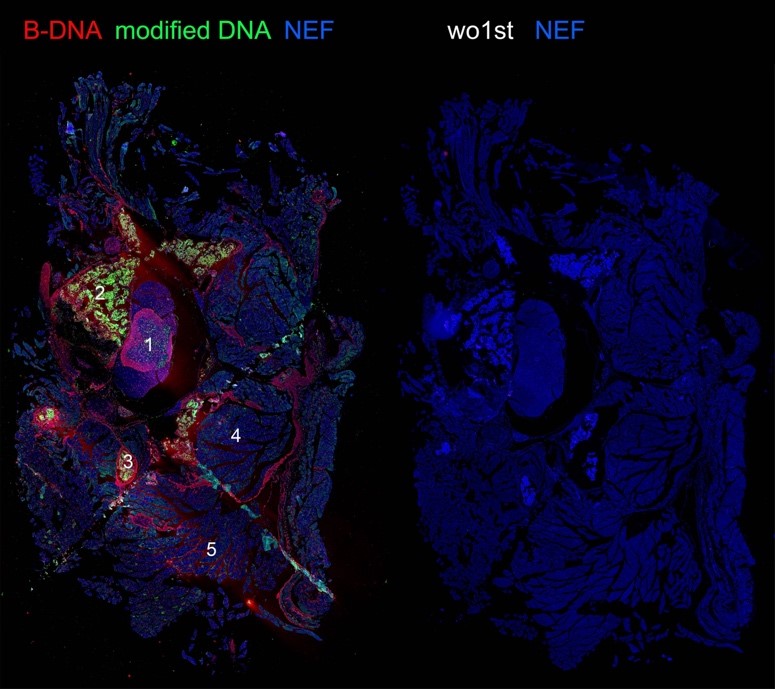 Figure 2: abundance of modified DNA in vertebral bodies upon experimental ischemia:
Figure 2: abundance of modified DNA in vertebral bodies upon experimental ischemia:(1) spinal cord (gray matter); (2) vertebral bodies (corticocancellous bone); (3) Processus transversus (4) paravertebral muscles; (5) muscles of the thoracic wall.
B-DNA (red) and modified DNA (green) were detected by antibodies; NEF: autofluorescence. Wo1st: negative controls.
TEAM
Project SPINET Team




| Dr. Annika Heuer | Dr. Jasmin Knopf | Prof. Michael Boettcher | Prof.Martin Herrmann |